“Mika helps me document my cancer, keeps me motivated, and acts as a link to my treatment team. I can warmly recommend it.”
Mika app user, from Playstore
“Great content that helps you engage with your disease in a positive way.”
“The app is very well-designed and informative. You feel very well-understood and supported in every situation.”
Mika app user, diagnosed with Breast Cancer
“It always does me good. In the beginning, I used it every day. Now, I only use it when I need it and it always gives me a boost. Thank you very much.”
“The app is very comprehensive with content for everyone. […] I think this app is and can be very helpful […]. Thank you.”
“During the worst period when I’d just learned I had cancer and hadn’t yet heard about psycho-oncology, Mika helped me not to give up.”
“Mika […] strengthens me mentally and helps me not to dwell [on my condition]. Mika makes me feel more optimistic, healthy, and resilient.”
“It’s good there’s space for the everyday challenges that arise when you have cancer. In general, I find the app great so I was happy to recommend it.”
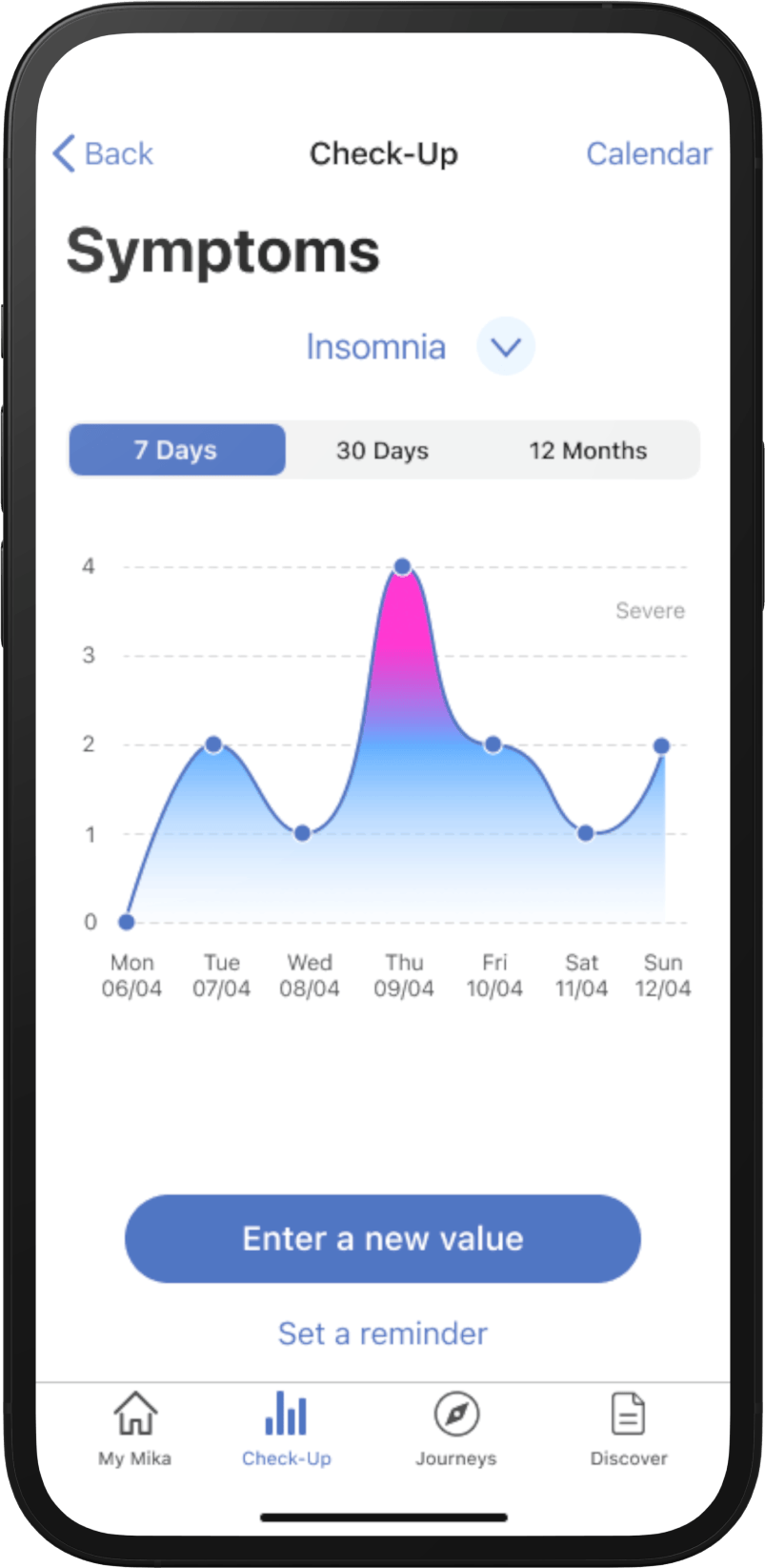
“Answers my questions. I really like the themed Journeys and relaxation content. I also find the diary very good for recording memories and feelings.”
“[I like] working through the themed Journeys that build on each other. The breathing and mindfulness exercises also benefit mind and body.”
“I don't feel depressed as often as I used to. When I feel anxious, I go through the relevant Journey and that helps.”
“Lots of additional information. I think the Journeys are very good. They help me reflect and and approach the disease differently.”
“The exercises have given me important tips for relaxation, managing my thoughts, and a more resilient sense of joy in my life.”
“Got back into mindfulness/relaxation exercises [...]. I can go into doctor's appointments more informed. The journal exercises are empowering.”
“It's a great support and provides stability. The themed Journeys, relaxation audio, and scientific articles are particularly helpful.”
“Mika [helped] me recover, both physically and mentally, through the worst time during and after cancer therapy. [It was] my constant companion.”
Mika app user, diagnosed with Ovarian Cancer
“Very informative and supportive. I look forward to continuing to use it.”
“I’m completely delighted and feel well looked after. [It helps me feel] informed and I have recommended it directly to my father.”
“Very good tips and suggestions for dealing better [...]. [I now have] a completely different feeling about the disease and how best to deal with it.”
“You always have everything to hand if you need to find information quickly. I also think it's very good that psychological needs are addressed.”
“Every day, you have the opportunity to get advice and read until you've internalized it. There's always an impetus toward positive things.”
“A lot of the information you get straight after an operation or during chemo just slips past you. It was very welcome to [...] to get further insight.”
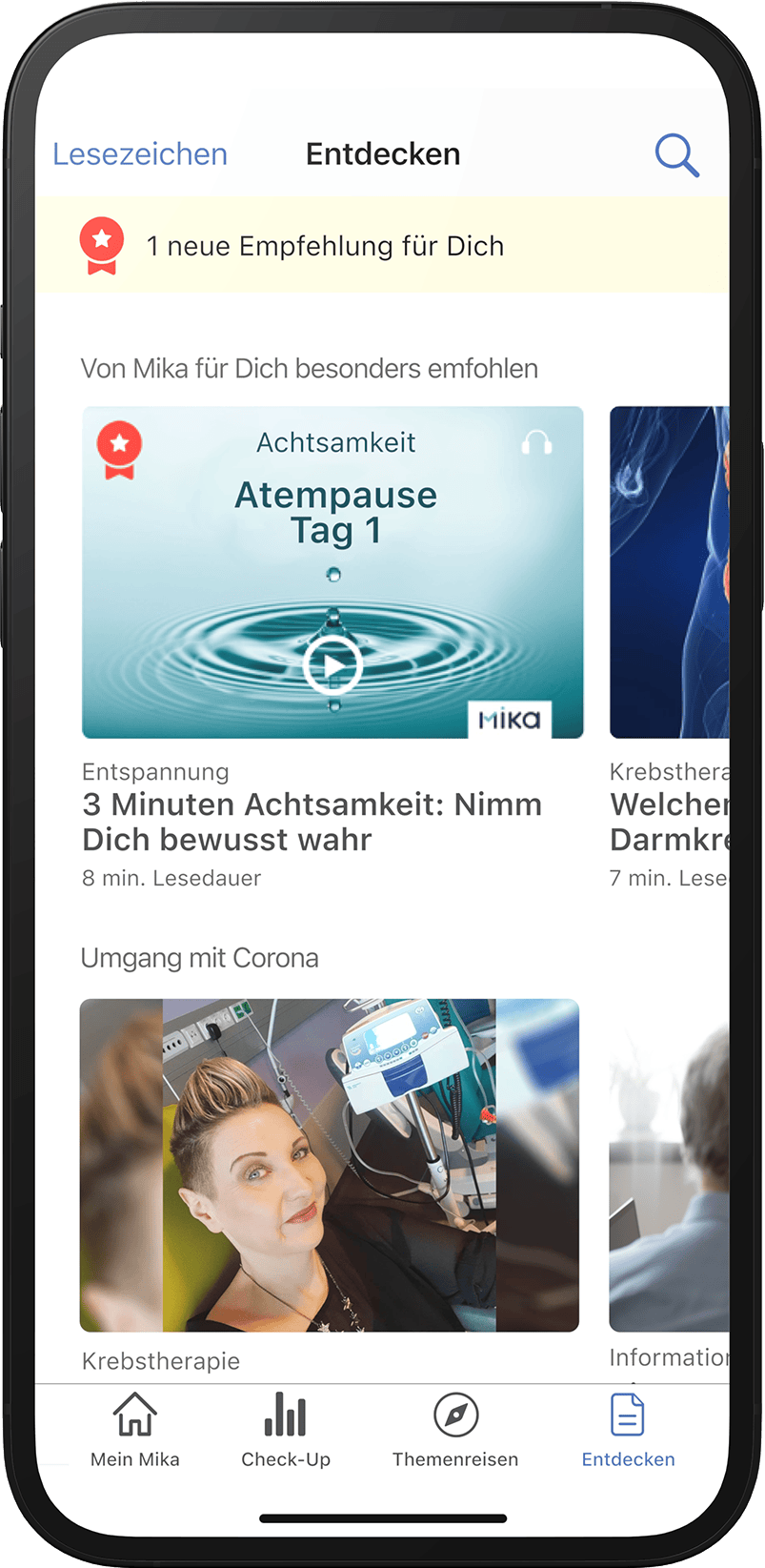
“Many articles in the ’Discover’ section really helped me to understand the disease and my situation. I devoured one article after another.”
“Regular reminders, good exercises with helpful hints, links to articles. Overall positive and the app is not annoying but motivating.”